EQUILIBRIUM
state IS reached when [reactants] & [products] ( constant over time
eq mixture
reacs & prods
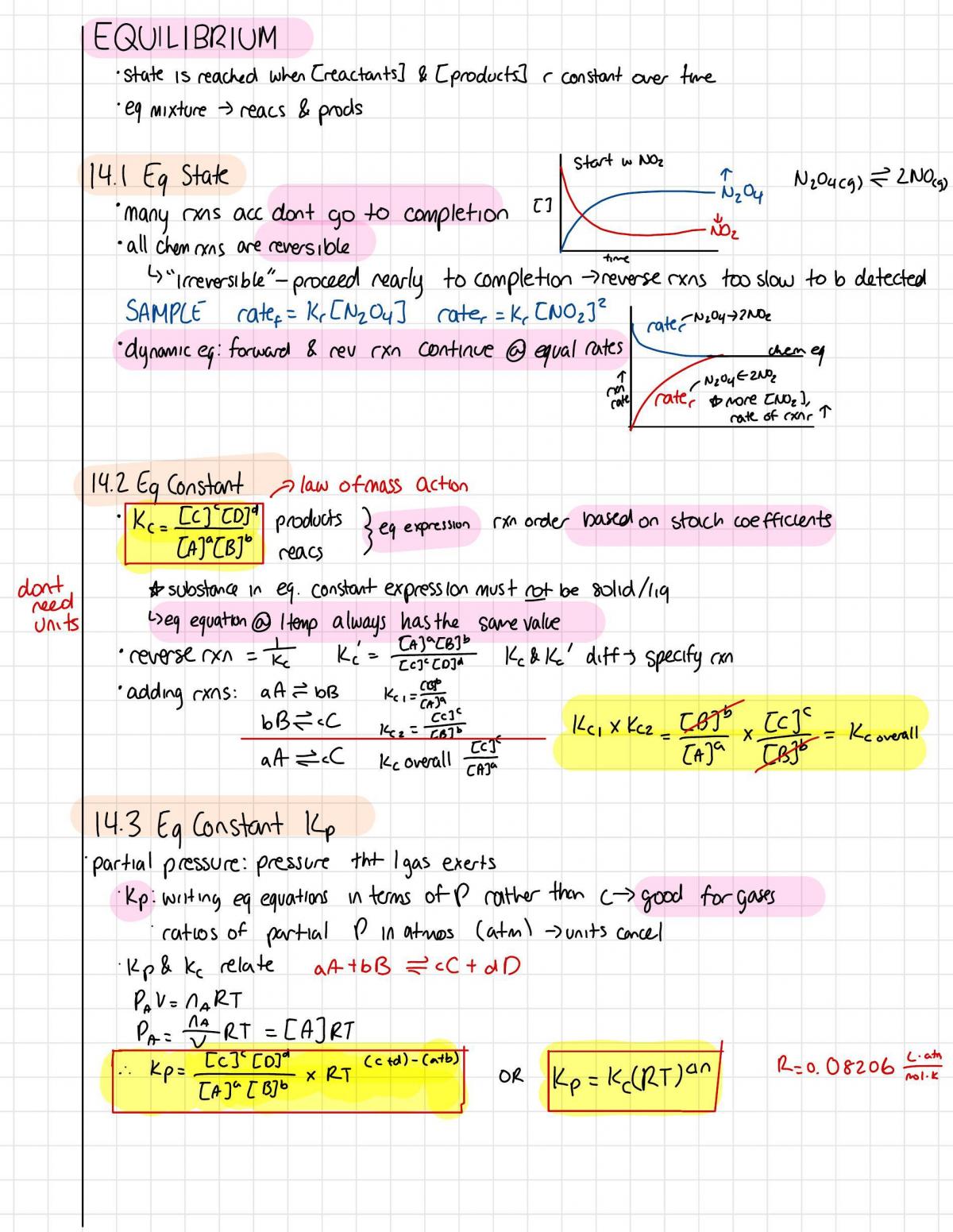
start 3 NO2
14.1 Eq State
N2O4
N2O4C9)
2NO(9)
many rxns acc dont go to completion
[]
NO2
all chen rxas are reversible
time
"Irreversible"- proceed rearly to completion
reverse rxns too slow to b detected
SAMPLE rate = Kr [N2O4] rater = K, [NO2]
rater
N2O4
2NO2
dynamic eg: forward & rev rxn continue @ equal rates
chem en
1
N2O4
2No2
(M)
cate
rater
more [NO2],
rate of exnr
14.2 Eq Constant
law ofmass action
Kc= = [C]°CD]"
products
eg expression rxn order based on stach coe fficients
[A]°[B]"
reacs
dont
need
substance in eq. constant expression must not be 80/12/119
Units
L)eq equation @ 1 temp always has the same value
=
Kc=
CA]a[B]b
reverse
ГХЛ
=
[c]'[D]' CD]"
Kc & Kg' diff
specify СХЛ
adding rxns: aA
bB
Kc, = CBP
bB
CC
Cc]c
Kc2 =
KcI X Kc2
CO75
[c]c
[C]
11
X
=
Kcoverall
aA
Y
kc overall
[A]a
CAJa
IBJb
14.3 Eq Constant Kp
partial pressure: pressure tht I gas exerts
Kp writing eq equations IN terms of P rather then
C
good for gases
ratios of partial P In atnos (atm)
units cancel
Kp& Kc relate aA +bB
cC + dD
PA = nA RT
NA
PA=
A=
>
RT = [A]RT
[c]' [0]
(ctd)-(atb)
kp=
"
x RT
OR
Kp = Kc(RT)an
R= O. 08206
C.ata
nol.k
[A Ja [B]b