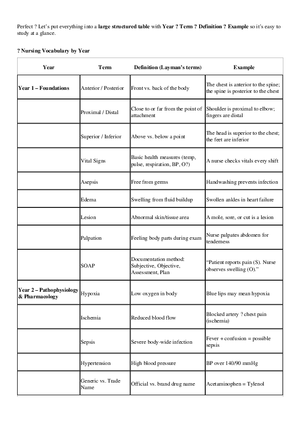
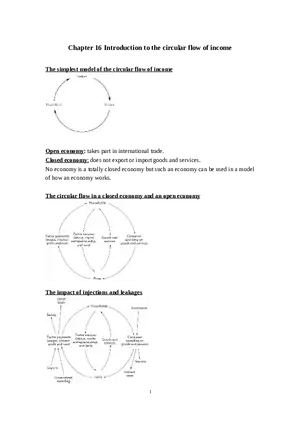
Assignment
Covalent Bonding Lewis Structures Webquest
-
University:
University of California, San Diego -
Course:
ECON 211 | Advanced Macroeconomics Academic year:
2025
-
Views:
0
Pages:
1
Author:
Cecilia Bittle
Related Documents
Report
Tell us what’s wrong with it:
Thanks, got it!
We will moderate it soon!
Report
Tell us what’s wrong with it:
Free up your schedule!
Our EduBirdie Experts Are Here for You 24/7! Just fill out a form and let us know how we can assist you.
Take 5 seconds to unlock
Enter your email below and get instant access to your document