Properties of Water
What Makes Water So Special?
Directions: Remember to answer the following questions using a different color font.
Why?
When you hear that NASA's space probes are looking for "evidence of life" on other planets, do you
know what that means? They are looking for evidence of liquid water. Water is fundamental for all life;
without it every living thing would die. Water covers about 70% of Earth's surface and it makes up
65-75% of our bodies (82% of our blood is water). Even if water might seem boring to you-no color,
taste, or smell-it has amazing properties that make it necessary for supporting life.
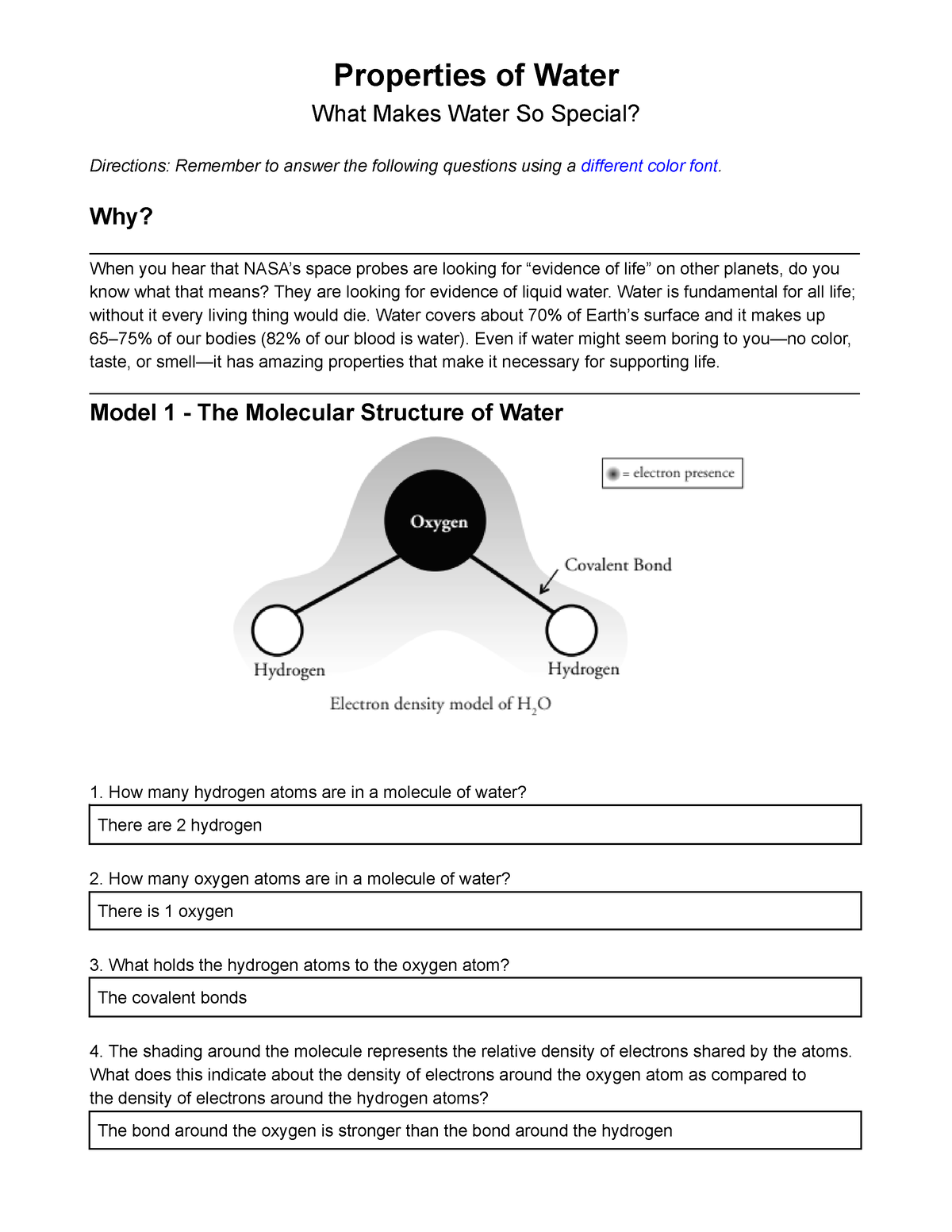
Model 1 - The Molecular Structure of Water
= electron presence
Oxygen
Covalent Bond
Hydrogen
Hydrogen
Electron density model of H2O
1. How many hydrogen atoms are in a molecule of water?
There are 2 hydrogen
2. How many oxygen atoms are in a molecule of water?
There is 1 oxygen
3. What holds the hydrogen atoms to the oxygen atom?
The covalent bonds
4. The shading around the molecule represents the relative density of electrons shared by the atoms.
What does this indicate about the density of electrons around the oxygen atom as compared to
the density of electrons around the hydrogen atoms?
The bond around the oxygen is stronger than the bond around the hydrogen